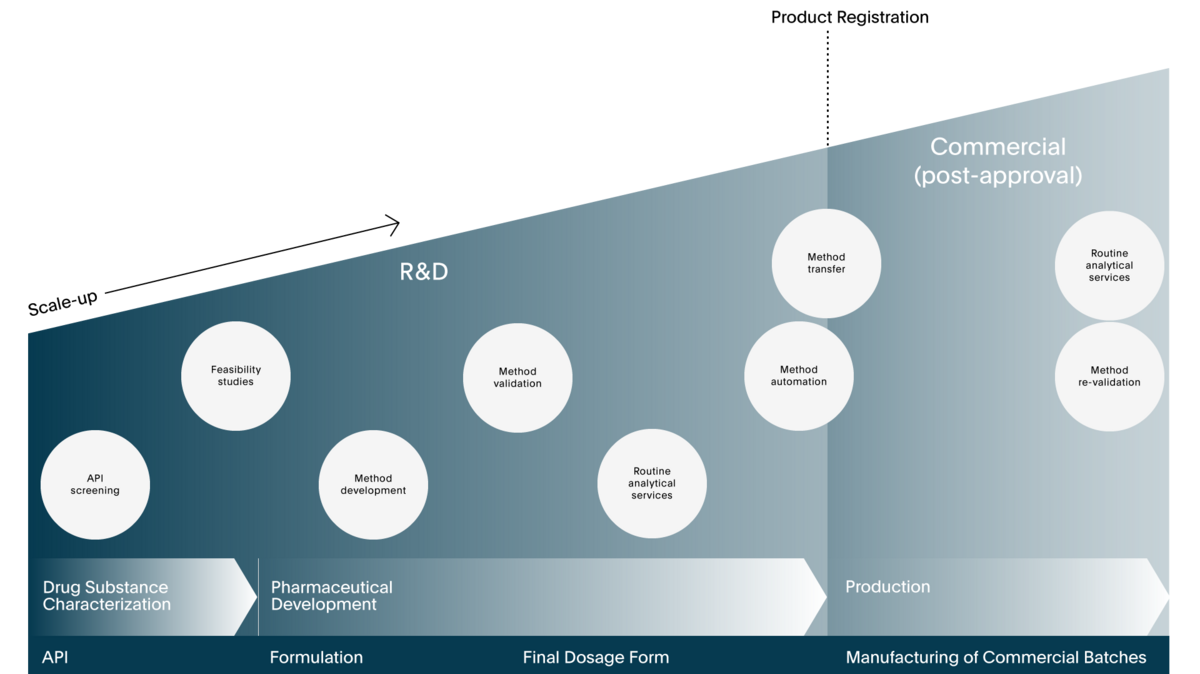
Comprehending the requirements of multiple disciplines is fundamental to our Pharma Services. Our experts in dissolution testing have experience with a wide range of issues, including developing a robust method, analysing samples using different analytical techniques, governing norms and regulations, differently automated instrumentation, characterising APIs, routine testing, stability testing, and more.To fully utilise its potential, one must have a solid understanding of the different sectors that affect results, such as API characteristics, formulation composition, manufacturing process, and biopharmaceutical performance prediction.

Dissolution Experts.
Our DNA is in-vitro dissolution testing. Being the only Contract Research Organisation (CRO) with a dissolution testing speciality, our team has a track record of successfully determining the best approach for a wide range of products, including implants, semi-solids, tablets, APIs, and many more.

Beyond Dissolution.
In-vitro dissolution testing has been Ortiv-Q3's area of expertise since 2019, however the company offers much more than "just" dissolution-related services. Our teams are proficient in many analytical techniques and have access to cutting-edge instrumentation for a wide range of test types, including stability testing and API characterization.

Quality System.
In accordance with US 21 CFR Parts 210, 211 and the guidelines of ICH Q10, the management of Ortiv-Q3 Research is dedicated to putting into place a Pharmaceutical Quality system that satisfies the relevant Good Manufacturing Practices criteria. Ortiv-Q3 Research is dedicated to the highest standards of professional conduct and customer service, utilising established protocols, techniques, and skilled and productive staff to deliver dependable, high-caliber outcomes on schedule.
One of three labs – worldwide.
In addition to Ortiv-Q3 in India, SOTAX has two other laboratories in Europe and the USA, all based on the same proven quality management system and offering first-class services in a GMP-certified and FDA-approved environment. Throughout the whole life cycle of your products, our local experts can help you, whether you need assistance with technological development or want to outsource regular testing.
Asia-Pacific
India
Asia-Pacific
India
Ortiv-Q3 Research Pvt. Ltd.
Ratna Business Hub, B-202-207, 2nd Floor
Near Sanathal Circle, Sarkhej - Bavla Highway
Ahmedabad, Pin 382 210, India
P Office +91 76 7670 7801
info@ortiv.in

Europe
France
Europe
France
SPS Pharma Services Sàrl.
3 rue Chateaubriand
45071 Orleans Cedex 2, France
P Office +33 2 34 59 72 61
pharmaservices@sotax.com

Americas
USA
Americas
USA
SOTAX Corporation
2400 Computer Drive
Westborough, MA 01581, USA
P Office +1 508 417 1112
sotaxusa@sotax.com